Most people are familiar with the mineral talc. It can be crushed into a white powder that is widely known as "talcum powder". This powder has the ability to absorb moisture, absorb oils, absorb odor, serve as a lubricant and produce an astringent effect with human skin. These properties make talcum powder an important ingredient in many baby powders, foot powders, first aid powders and a variety of cosmetics.
A form of talc known as "soapstone" is also widely known. This soft rock is easily carved and has been used to make ornamental and practical objects for thousands of years. It has been used to make sculptures, bowls, countertops, sinks, hearths, pipe bowls and many other objects. Although talcum powder and soapstone are two of the more visible uses of talc they account for a very small fraction of talc consumption. Its hidden uses are far more common. Talc's unique properties make it an important ingredient for making ceramics, paint, paper, roofing materials, plastics, rubber, insecticides and many other products.
Talc is the softest mineral (occupying place 1 in the Mohs` hardness scale) known on our planet earth. It is lamellar, platy, organophilic, water repellent with special thermal and mechanical properties. It has a high capacity for absorbing organic substances. It is also acid & alkali-resistant, chemically inert and non-toxic. Talc has neither aroma nor taste. This makes it a very essential mineral in our day-to-day life. Talc is a hydrated magnesium silicate [chemical formula is Mg3Si4O10(OH)2 ]. Also known as Steatite. It is the main component of soapstone. Its crystals usually develop massive, leafy aggregates with laminar particles.

Its silicate layers lie on top of one another without having a chemical bond but are bound to each other by weak Vander Waals forces. This structure gives talc the platy appearance and its characteristic greasy or soapy feeling – hence the name "soapstone”. In its pure form, talc is colorless or appears white, and often it has a mother-of-pearl sheen. When it contains other substances, it can also appear light grey, green, yellowish or pink.
Formation of talc / soapstone :
 Talc can be formed in different geological environments or processes. Several types of talc deposits may be distinguished according to the present composition and parent rock from which they are derived. There are two types of talc deposits which contribute to the world's talc production are described below:
Talc can be formed in different geological environments or processes. Several types of talc deposits may be distinguished according to the present composition and parent rock from which they are derived. There are two types of talc deposits which contribute to the world's talc production are described below:
1. Dolomite-hosted talc and talc-chlorite rocks :
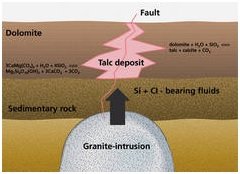
The talc is formed by the alteration of sedimentary magnesium carbonate rocks (dolomite and magnesite) at elevated temperature and pressure below the earth's surface. While the magnesium is fixed in situ, the silica is transported by silica-containing hot fluids to react with the Magnesium- bearing carbonates to form talc. These altered rocks are then talc-rich dolomites or magnesites. This type of deposits usually deliver the massive talc accompanied by carbonates, chlorite and some quartz. The mineral composition is generally 30 - 100% talc, 0 - 70% chlorite/carbonates and 0.1 - 0.5% quartz.
2. Ultramafic-hosted talc magnesite rocks :

These talc deposits result from hydrothermal alteration of magnesium-rich magmatic parent rocks. They are called ultramafic rocks while they consist mostly of mafic (dark Magnesium-rich) minerals. Alteration process is two-fold: first hydration of these mafic minerals such as olivine or pyroxene by H2O influx into serpentine which is a hydrated Magnesium-silicate. Second step is alteration of serpentine into talc and magnesite by CO2- addition. This kind of talc deposits occur in Finland, Norway, Sweden, Canada and Russia.
